 |  |  |
CLL NOTES:
RAI STAGING:
STAGE 0 (LOW RISK);Manifestation:lymphocytosis; Median Survival:>10years
STAGE I (INTERMEDIATE RISK); Manifestation: Lympahdenopathy; Median Survival:9 years
STAGE II (INTERMEDIATE RISK);Manifestation: Splenomegaly, lymphadenopathy, or both; Median survival:7 years
STAGE III (HIGH RISK); Manifestation: anemia,organomegaly, or both; Median survival:5 years
STAGE IV (HIGH RISK); Manifestation: anemia or thrombocytopenia, + organomegaly; median survival: 5 years
---------------------------------------------------------------------------------------
BINET STAGING
STAGE A (LOW RISK); Manifestations: lymphocytosis or >3 lymphoid areas enlarged. Median Survival: >10 years
STAGE B (INTERMEDIATE RISK); Manifestations: >3 lymphoid areas enlarged; Median Survival:7 years
STAGE C (HIGH RISK); Manifestations: anemia, thrombocytopenia, or both; Median Survival: 5 years
---------------------------------------------------------------------------------------
Genomic Aberrations & Survival in CLL:
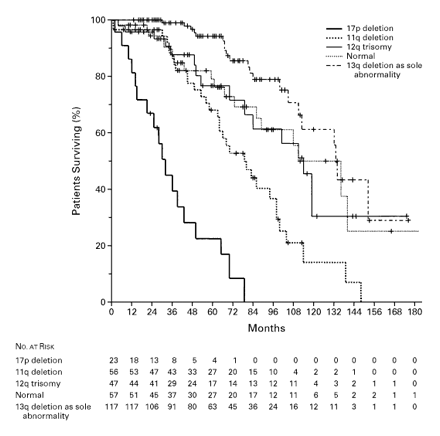
Chromosomal aberrations were detected in 268 of 325 cases (82%). The most frequent changes were a deletion in 13q (55%), a deletion in 11q (18%), trisomy of 12q (16%), a deletion in 17p (7%), and a deletion in 6q (6%). Five categories were defined with a statistical model: 17p deletion, 11q deletion, 12q trisomy, normal karyotype, & 13q deletion as the sole abnormality; the median survival times for patients in these groups were 32, 79, 114, 111, & 133 months, respectively. Patients in the 17p- and 11q-deletion groups had more advanced disease than those in the other three groups. Patients with 17p deletions had the shortest median treatment-free interval (9 months), and those with 13q deletions had the longest (92 months). In multivariate analysis, the presence or absence of a 17p deletion, the presence or absence of an 11q deletion, age, Binet stage, the serum LDH level, and the WBC count gave significant prognostic information.
REF:NEJM 343:1910, 2000

|



